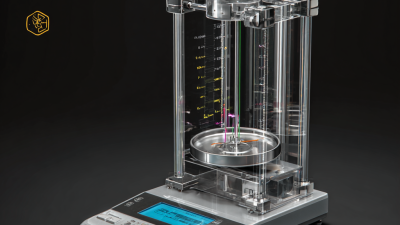
In laboratory settings, precise measurements are crucial for successful experimentation and research. The importance of accurate weighing cannot be overstated, as errors in measurement can lead to flawed results and compromised data integrity. According to a report by the National Institute of Standards and Technology (NIST), an error as small as 0.1 milligrams can significantly affect the outcomes of sensitive applications, particularly in fields such as pharmaceuticals and biotechnology. This is where an **Analytical Balance** becomes indispensable.
An Analytical Balance is designed to deliver measurements with a readability of 0.0001 grams, ensuring the highest level of precision required in most scientific studies. A recent survey published by the International Labmate indicated that laboratories utilizing high-accuracy equipment reported better reproducibility and reliability in their results, with nearly 75% of researchers advocating for the use of an Analytical Balance over conventional methods for critical weighing tasks. By investing in advanced weighing technology, laboratories can enhance their measurement accuracy, streamline their workflows, and ultimately contribute to more reliable scientific advancements.

Accurate measurements are critical in laboratory settings, where the precision of data can influence the outcomes of experiments and research. The American National Standards Institute (ANSI) suggests that an analytical balance can help achieve a readability of 0.0001g, which is essential for experiments requiring exact mass measurement. In pharmaceuticals, for example, even a slight deviation in ingredient measurement can lead to ineffective formulations or potentially harmful effects, highlighting the need for precise tools in these environments.
Moreover, studies indicate that laboratory errors—many stemming from inadequate measurement practices—can lead to significant financial losses, estimated at over $50 billion annually in the U.S. alone. A report from the Laboratory Safety Institute emphasizes that nearly 20% of these errors are directly attributed to instruments that do not meet the necessary accuracy requirements. By utilizing an analytical balance, labs can minimize variability in results, thus enhancing the reliability and validity of experimental data. This technological investment not only streamlines processes but also promotes a culture of precision, essential in scientific inquiry across various fields, including chemistry and biochemistry.
Analytical balances are precision instruments designed for the most accurate measurement of mass in laboratory settings. They are specifically engineered to provide measurements with a readability of 0.0001 grams or better, allowing researchers to quantify minute quantities of substances. This level of precision is essential in fields such as chemistry, biology, and materials science, where even the smallest deviations in mass can lead to significant errors in experimental results.
The functionality of analytical balances goes beyond just superior accuracy; they typically feature draft shields to minimize the influence of air currents, which can affect measurements. Many models utilize advanced weighing technology to ensure consistent performance, including internal calibration systems that automatically adjust for environmental changes, such as temperature and humidity fluctuations. Moreover, their user-friendly interfaces often include digital readouts and data connectivity options, facilitating seamless integration with laboratory information management systems, thereby enhancing workflow efficiency. The combination of these features makes analytical balances indispensable tools for any laboratory aiming for precise and reliable measurement results.
| Feature | Description | Importance |
|---|---|---|
| Capacity | Typically ranges from 10 g to 200 g | Ensures accuracy for various sample sizes |
| Readability | Usually 0.1 mg or better | Crucial for precise measurements |
| Calibration | Automatic or manual calibration options | Maintains measurement accuracy over time |
| Pan Size | Larger pans may accommodate bigger samples | Facilitates weighing of larger containers |
| Draft Shield | Minimizes air currents during measurement | Enhances measurement stability |
| Connectivity | USB or RS232 for data transfer | Allows integration with lab systems |
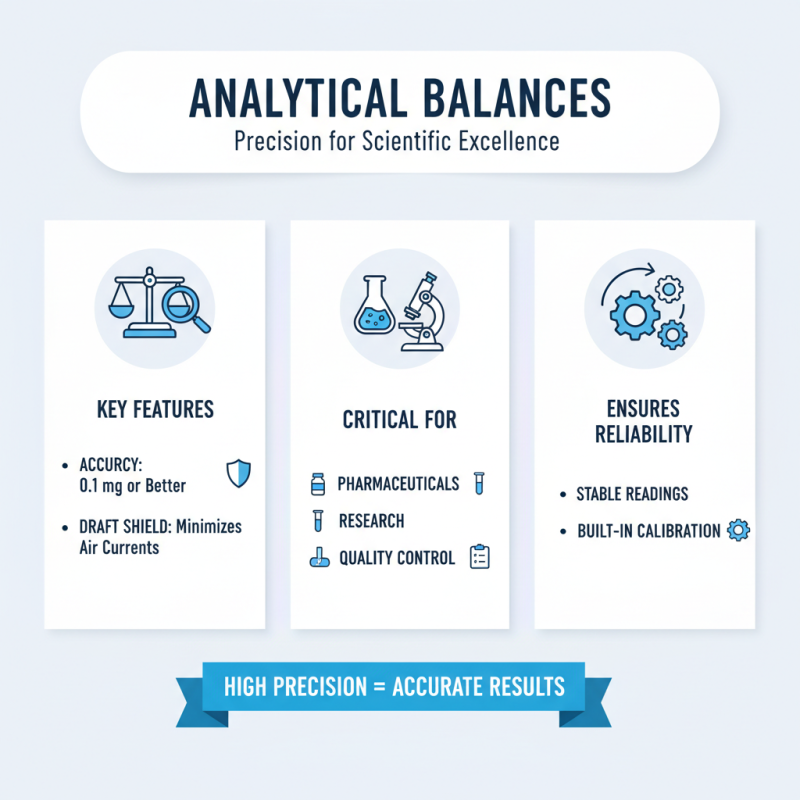
Analytical balances are essential instruments in laboratories that demand high precision in measurements. One of the key features of an analytical balance is its ability to measure with an accuracy of 0.1 mg or better. This high level of precision is vital for applications such as pharmaceuticals, research, and quality control, where even the slightest deviation can lead to significant errors in results. The draft shield included in many models minimizes the impact of air currents, providing stable and reliable readings, while the built-in calibration mechanisms ensure that the balance maintains its accuracy over time.
Another important aspect of analytical balances is their sensitivity to environmental factors. Many modern balances are equipped with automatic sensors that compensate for temperature and humidity fluctuations, further enhancing measurement reliability. The readability display allows users to easily interpret results, often providing features such as tare functions, conversion to different units, and data storage capabilities. Together, these features make analytical balances indispensable tools in any laboratory setting where precision and accuracy are paramount for successful experimentation and analysis.
Analytical balances are indispensable tools in various scientific fields, enabling precise measurements essential for research and experimentation. In chemistry, these balances are crucial for formulating compounds and analyzing substances. Accurate weighing is vital for reaction stoichiometry, where even slight deviations can lead to significant errors in outcomes. Similarly, in pharmaceuticals, analytical balances ensure the accurate dosage of active ingredients, which is critical for efficacy and safety.
In the field of biology, analytical balances support various applications, from preparing samples for tissue culture to quantifying biochemical substances. Accurate measurements help ensure reproducibility in experiments, which is essential for validating results. Environmental science also benefits from analytical balances when measuring pollutants and toxins, which can impact both ecological studies and public health assessments.
Tips: Always calibrate your analytical balance regularly to maintain accuracy. Additionally, minimize air currents and vibrations in the workspace by using draft shields and placing the balance on a stable, level surface. Finally, always handle samples with precision using gloves or tweezers to avoid contamination, which can lead to inaccurate results.
Proper maintenance and calibration of analytical balances are critical to achieving reliable and precise measurements in laboratory settings. Regular maintenance involves keeping the balance clean and free of any debris that could interfere with measurements. This includes ensuring that the weighing pan is free of residues and that the surrounding area is tidy and organized. Additionally, environmental factors such as drafts or vibrations can impact the accuracy of the readings, so it's essential to place the balance in a stable environment, ideally within a designated weighing enclosure.
Calibration is equally important, as it ensures that the balance provides accurate readings over time. Calibration should be performed regularly using certified calibration weights that fall within the range of the balance's capacity. This process helps identify and rectify any deviations from true values, ensuring that the balance operates within specified tolerances. Documenting calibration dates and results is vital for maintaining compliance with quality standards and for tracking any changes that might affect measurement accuracy. By prioritizing consistent maintenance and thorough calibration, laboratories can guarantee the reliability of their analytical balances and the validity of their experimental results.