An Analytical Balance is vital in laboratories, ensuring precision in measurements. Dr. Emily Chen, a leading expert in analytical instrumentation, states, “Accuracy in weighing is crucial for reliable results.” This highlights the critical role that an Analytical Balance plays in various scientific fields.
These balances are designed to measure small masses with high precision. Their sensitivity often reaches up to 0.0001 grams. In many experiments, even slight variations can lead to significant discrepancies. Therefore, understanding how an Analytical Balance operates becomes essential for researchers.
Despite its importance, users sometimes overlook proper calibration. Regular maintenance is key for accurate readings. The environment can also affect results, such as drafts or temperature changes. This means that achieving perfect measurements often requires effort. Testing one’s techniques with an Analytical Balance can lead to reflection on personal practices in the lab.

An analytical balance is a precision instrument used for measuring mass with a high degree of accuracy. Unlike standard scales, it can measure very small amounts of substance, often down to 0.0001 grams. This sensitivity makes it essential in laboratories and research settings where precision is crucial.
The operation of an analytical balance relies on a principle called electromagnetic force restoration. When a sample is placed on the balance, it triggers a system that compensates for the weight. This results in a reading that is extremely accurate. The design includes a draft shield that minimizes disturbances from air currents, ensuring even the slightest changes in mass are detected.
Using an analytical balance requires careful handling. Users must ensure the balance is calibrated regularly. Any obstruction or dust can lead to errors. A stable environment free from vibrations is also vital. Each of these factors contributes to the reliability of measurements. Even with modern technology, human error can still affect outcomes. Balancing precision with practical use is an ongoing challenge for many researchers.
This bar chart displays the sensitivity levels (in grams) of different types of analytical balances commonly used in laboratory settings. Sensitivity is a critical factor in determining the accuracy of measurements in quantitative analysis.
An analytical balance is a precise instrument used for measuring small masses with high accuracy. Its key components distinctly contribute to its exceptional performance. The weighing pan is critical. Typically, it has a diameter of about 80-120 mm, which allows for precise sample placement. The pan is not just for holding samples; its design helps minimize air drafts.
Another essential component is the electromagnetic force restoration system. This system applies a magnetic force to counterbalance the weight of the sample. Reports indicate that this mechanism can measure down to 0.0001 grams. In environments where vibrations are an issue, the balance further incorporates a draft shield. This feature is vital as even slight drafts can distort readings.
The display and controls are equally important. Many analytical balances use digital interfaces. A user-friendly interface makes data interpretation easier. However, relying solely on the display can lead to overlooked errors. Maintaining regular calibration is necessary but often neglected. Regular checks ensure accuracy, yet many users forget this step, leading to inconsistent results. Balancing precision with user diligence is crucial for optimal performance.
| Component | Description | Function |
|---|---|---|
| Weighing Pan | A flat surface where samples are placed for weighing. | Supports the sample during measurement. |
| Draft Shield | Encloses the weighing pan to minimize air currents. | Ensures accurate readings by reducing disturbances. |
| Load Cell | The sensor that converts weight into an electrical signal. | Enables precise measurement of weight. |
| Display Unit | Screen that shows the weight measurement. | Presents real-time weight information to the user. |
| Calibration Weights | Standard weights used to calibrate the balance. | Ensures accuracy and precision in measurements. |
| Electronics | Components that process signals from the load cell. | Controls balance functions and data output. |
Analytical balances are precision instruments designed for measuring small masses. They provide an accuracy that standard scales cannot match. These balances operate in draft-free environments to prevent errors. A draft shield often encloses the weighing chamber. This feature is crucial for ensuring stable readings.
Calibration is essential for maintaining precision. Regular checks against known standards help identify discrepancies. Environmental factors like humidity and temperature can affect readings. Users must be vigilant about these conditions. Even minor factors can lead to significant errors.
Training in the proper use of analytical balances is vital. Inexperienced users might not handle samples correctly. Misplacing a sample or not zeroing the balance can cause inaccurate results. Continuous learning and adjustment improve overall accuracy. It's important to reflect on one's technique and make improvements as needed.
Analytical balances are critical tools in laboratories. These precise instruments measure mass with high accuracy. Common applications include drug formulation, chemical analysis, and quality control. They offer great precision and can measure small quantities effectively, often down to 0.1 milligram.
In pharmaceuticals, analytical balances help ensure correct dosages. Accurate measurements are vital for patient safety. A small error can lead to significant consequences. In chemical research, these balances assist in preparing reagents. Precise mass affects reaction outcomes, influencing findings.
Quality control labs use analytical balances to assess product consistency. Every batch must meet specific standards. If the measurements are off, the entire batch may be compromised. Users must keep balances calibrated. Regular checks are necessary to maintain accuracy. This leads to reliable results and trustworthy research.
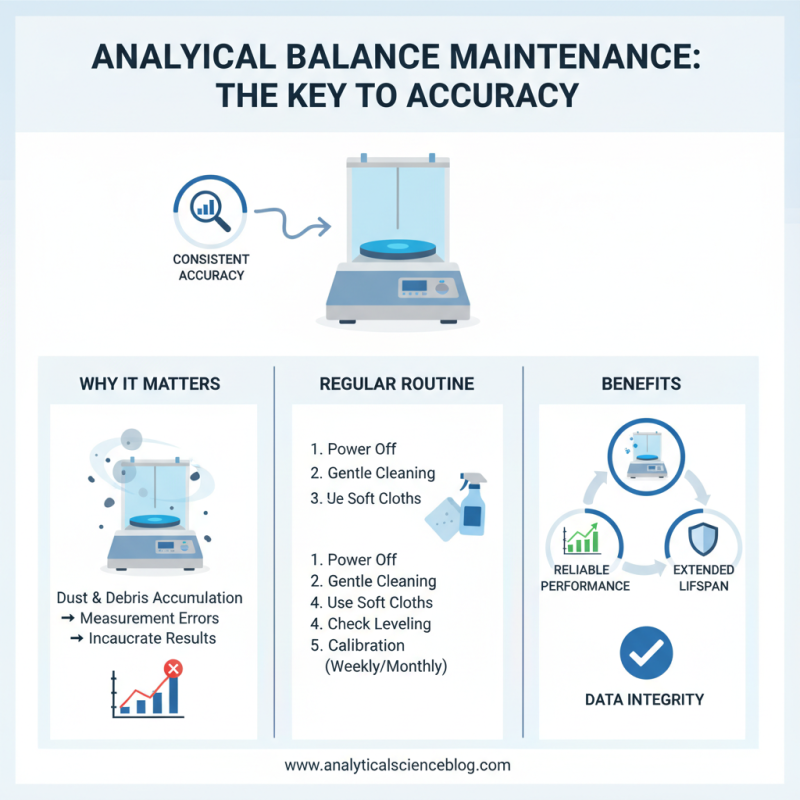
Maintaining an analytical balance is crucial for accuracy. Regular maintenance ensures consistent performance. Dust and debris can accumulate, affecting measurements. It's important to regularly clean the balance's surface. Use soft cloths to avoid scratches.
Calibration is equally essential. It helps to confirm that the balance provides precise readings. Calibration can be done using standard weights. These should be of known mass and regularly checked. A balance can drift over time, so retraining is necessary.
The environment plays a role too. Vibrations and air currents can impact results. Positioning the balance on a stable table is recommended. Humidity and temperature should also be controlled. Sometimes, users forget these factors, leading to significant errors. An analytical balance is sensitive and requires care for optimal operation.






